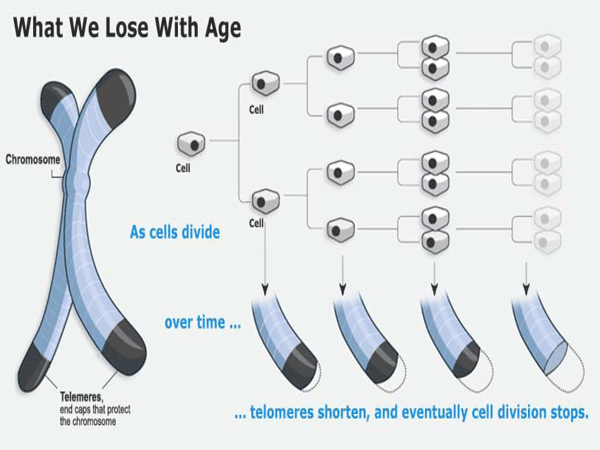
How to engineer negligible senescence in humans part VII Biology Diagrams Since then, great advances have been made in the understanding of how telomeres are able to signal the senescence arrest. These mechanisms are of particular importance in the field of ageing, since cellular senescence, driven by telomere dysfunction, has been shown to be a causal driver of ageing and age-related pathology (van Deursen, 2014). 1.1.
VSMCs in human atherosclerotic plaques show cellular senescence markers and telomeres that are markedly shorter than those in unaffected vessels from the same individual 115. Telomere dysfunction induced by VSMC-specific expression of mutant TRF2 is sufficient to increase atherosclerosis 116,117.
FBW7 Mediates Senescence and Pulmonary Fibrosis through Telomere Uncapping Biology Diagrams
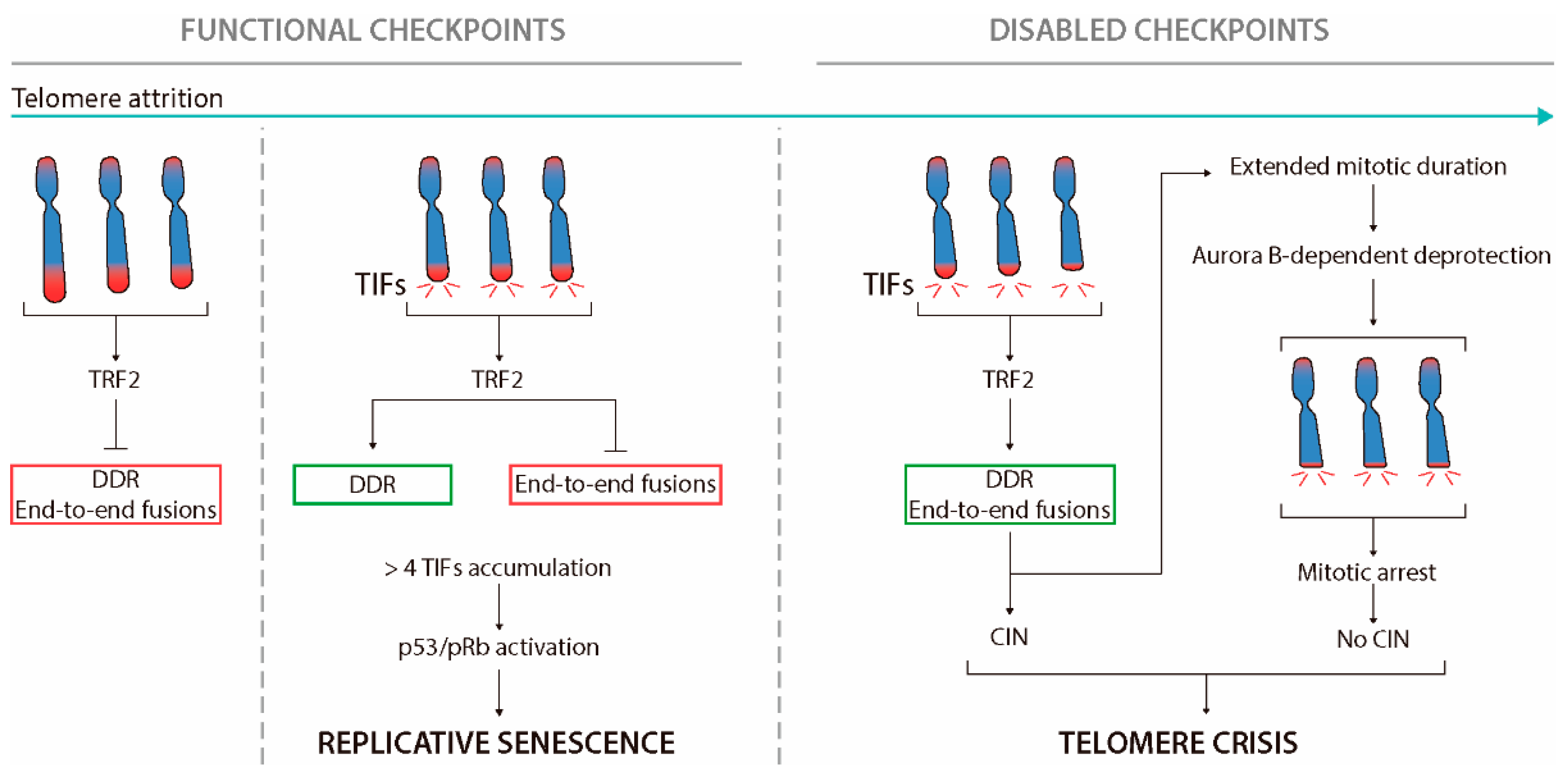
Here, we report that in response to radiation, oxidative stress, or bleomycin, the E3 ubiquitin ligase FBW7 mediates cell senescence and tissue fibrosis through telomere uncapping. FBW7 binding to telomere protection protein 1 (TPP1) facilitates TPP1 multisite polyubiquitination and accelerates degradation, triggering telomere uncapping and DNA Telomere uncapping with advancing age might lead to P53/P21-induced senescence and subsequent inflammation in arteries. Although age-related telomere uncapping has not been assessed in noncultured human tissues, telomere shortening has been shown to occur over time in most human somatic tissues (23, 30, 47), including arteries (12, 41, 44). Environmental stressors such as bacterial toxins and radiation can cause premature aging and fibrosis. Wang et al. reveal that in mice exposed to radiation or the bacterial toxin bleomycin, the tumor suppressor protein FBW7 marks the telomere protein TPP1 for degradation, triggering telomere uncapping and shortening, cancer or stem cell aging, and pulmonary fibrosis. Targeting FBW7 by an 8-mer

Next, we investigated the mechanisms by which FBW7 mediates stress-induced cell senescence. The telomere shelterin protein TPP1 was found to be significantly unstable in the presence of H 2 O 2, BLM, or IR, undergoing rapid degradation in human pulmonary A549 cells (Figure 2 A). Having confirmed the specificities of detecting shelterin proteins TPP1, TIN2, TRF1, TRF2, and Rap1 using Interestingly, unlike telomere length, telomere uncapping was found to strongly correlate to markers of p53/p21-associated senescence in arteries from healthy young and old individuals. 21 Furthermore, in comparison with healthy old controls, age-matched hypertensive individuals have greater p53/p21-dependent senescence and telomere uncapping In this review, we describe how telomere uncapping potentially leads to age-related vascular dysfunction and increased cellular senescence, oxidative stress, and inflammation. Importantly, we present evidence to argue that telomere uncapping is more biologically relevant than telomere shortening and a better marker of vascular aging and target
